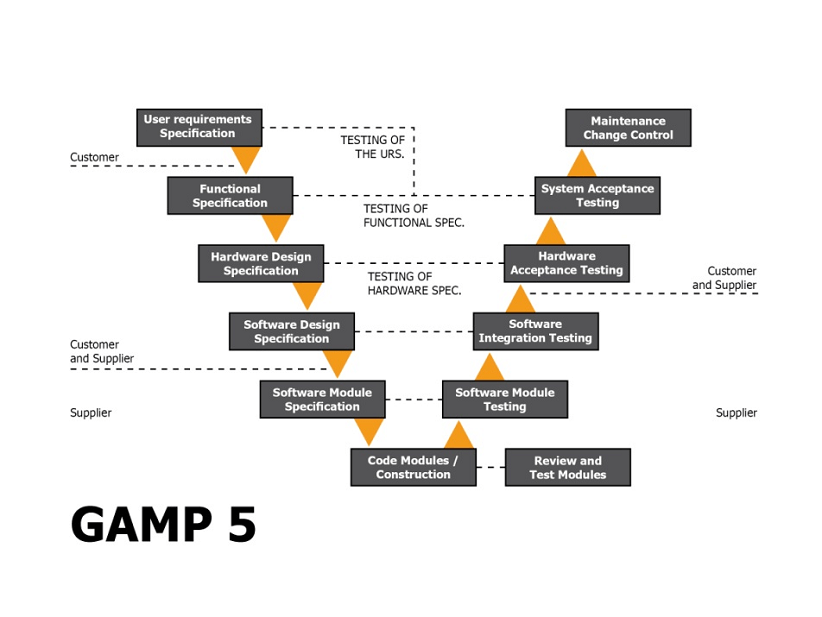
Gamp 5 Training Course
Gamp 5 Training Course - This classroom or online course has been updated to include the new revised gamp ®. The gamp5 methodology serves as a crucial guide in ensuring the validation and compliance of computerized systems and to facilitate regulatory standards. This course delves into the intricacies of gamp5, with a focus on its second edition. Gamp 5, annex 11/part 11 basic principles training course. Experts from the pharmaceutical industry and from the gamp® committee will show you efficient ways to validate your computerised systems. Experts from the pharmaceutical industry and from the gamp® committee will show you efficient ways to validate your computerised systems. Critical thinking in and about the 2nd edition This classroom or online course has been updated to include the new revised gamp ®. The gamp® essentials certificate program provides fundamental training on good manufacturing practices (gmps) for computerized systems in the pharmaceutical and healthcare industries. Basic principles of computerized systems compliance: This classroom or online course has been updated to include the new revised gamp ®. Critical thinking in and about the 2nd edition Experts from the pharmaceutical industry and from the gamp® committee will show you efficient ways to validate your computerised systems. How has the 2nd edition been affected by new technological developments; Upon completion of this course you will be able to, understand the importance of computer system validation, the regulations and standards behind csv, recommendations and best practices for csv, understand the role of gamp®5 (gamp5) and the competence required for a validation team. This classroom or online course has been updated to include the new revised gamp ®. Gamp 5, annex 11/part 11 basic principles training course. We tailor the courses to meet your companies needs, enable your team to cover the technical syllabus of the course while also working on some of your real operational or project issues in the privacy of your own team and offices. The gamp® essentials certificate program provides fundamental training on good manufacturing practices (gmps) for computerized systems in the pharmaceutical and healthcare industries. 'compliant gxp computerized systems' and 'gamp 5 good practice guide ‘enabling innovation’. Basic principles of computerized systems compliance: Implementing the gamp® 5 guide. The event will be in chicago, illinois, usa on thursday, 25 january 2024. 5 documents can be found on the. Basic principles of computerized systems compliance: The gamp5 methodology serves as a crucial guide in ensuring the validation and compliance of computerized systems and to facilitate regulatory standards. 'compliant gxp computerized systems' and 'gamp 5 good practice guide ‘enabling innovation’. The event will be in chicago, illinois, usa on thursday, 25 january 2024. Basic principles of computerized systems compliance: 5 documents can be found on the. 5 documents can be found on the. This course delves into the intricacies of gamp5, with a focus on its second edition. This live online training is directed towards specialists and executives in the pharmaceutical industry entrusted with the planning, implementation and evaluation of the validation of computerised systems. What are the changes in the main part? Critical thinking in. What are the changes in the main part? Applying the gamp ® 5 guide: The basis of the training will be the current requirements for the validation of computerised systems like gamp® and their gxporiented application in practice. #performancevalidation is proud to sponsor the upcoming great lakes chapter gamp americas forum on january 25, 2024, in chicago, illinois! Implementing the. Applying the gamp ® 5 guide: Basic principles of computerized systems compliance: The event will be in chicago, illinois, usa on thursday, 25 january 2024. #performancevalidation is proud to sponsor the upcoming great lakes chapter gamp americas forum on january 25, 2024, in chicago, illinois! The gamp5 methodology serves as a crucial guide in ensuring the validation and compliance of. Upon completion of this course you will be able to, understand the importance of computer system validation, the regulations and standards behind csv, recommendations and best practices for csv, understand the role of gamp®5 (gamp5) and the competence required for a validation team. The course covers recommended good practice based on a lifecycle approach for the development and management of. The event will be in chicago, illinois, usa on thursday, 25 january 2024. Applying the gamp ® 5 guide: Fundamental principles of compliance for computerized systems: Implementing the gamp® 5 guide. What are the changes in the management and operation appendices? Upon completion of this course you will be able to, understand the importance of computer system validation, the regulations and standards behind csv, recommendations and best practices for csv, understand the role of gamp®5 (gamp5) and the competence required for a validation team. We tailor the courses to meet your companies needs, enable your team to cover the technical syllabus. The gamp5 methodology serves as a crucial guide in ensuring the validation and compliance of computerized systems and to facilitate regulatory standards. Basic principles of computerized systems compliance: Applying the gamp ® 5 guide: Fundamental principles of compliance for computerized systems: Basic principles of computerized systems compliance: Get to know the differences between gamp®5 and gamp®5 2nd edition. 'compliant gxp computerized systems' and 'gamp 5 good practice guide ‘enabling innovation’. The event will be in chicago, illinois, usa on thursday, 25 january 2024. Critical thinking in and about the 2nd edition Applying the gamp ® 5 guide: This classroom or online course has been updated to include the new revised gamp ®. 5 documents can be found on the. Fundamental principles of compliance for computerized systems: Applying the gamp ® 5 guide: This classroom or online course has been updated to include the new revised gamp ®. This fundamental online course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried, tested, and internationally recognized methods of meeting those requirements. Experts from the pharmaceutical industry and from the gamp® committee will show you efficient ways to validate your computerised systems. #performancevalidation is proud to sponsor the upcoming great lakes chapter gamp americas forum on january 25, 2024, in chicago, illinois! This course delves into the intricacies of gamp5, with a focus on its second edition. We tailor the courses to meet your companies needs, enable your team to cover the technical syllabus of the course while also working on some of your real operational or project issues in the privacy of your own team and offices. 'compliant gxp computerized systems' and 'gamp 5 good practice guide ‘enabling innovation’. Critical thinking in and about the 2nd edition Get to know the differences between gamp®5 and gamp®5 2nd edition. This live online training is directed towards specialists and executives in the pharmaceutical industry entrusted with the planning, implementation and evaluation of the validation of computerised systems. The basis of the training will be the current requirements for the validation of computerised systems like gamp® and their gxporiented application in practice. Applying the gamp ® 5 guide:GAMP 5® Innovation in a Flexible Manner LearnGxP Accredited Online
Gamp 5 training importance
GAMP®5 Roles and Responsibilities Simplified Process Owner [Video
Training in Computer System Validation in Copenhagen
GAMP®5 Roles and Responsibilities Simplified Process Owner [Video
GAMP 5 Training getting closer Lemontree
GAMP5 Training Release of new edition with important updates! Lemontree
Gamp 5 PDF
Computerised Systems Validation GAMP 5 Training Course
ISPE GAMP 5 Software Categories Hardware & Software LearnGxP
Basic Principles Of Computerized Systems Compliance:
This Online Training Course Is Directed Towards Specialists And Executives In The Pharmaceutical Industry Entrusted With The Planning, Implementation And Evaluation Of The Validation Of Computerised Systems.
Basic Principles Of Computerized Systems Compliance:
The Event Will Be In Chicago, Illinois, Usa On Thursday, 25 January 2024.
Related Post: