Regulatory Affairs Courses Near Me
Regulatory Affairs Courses Near Me - Regulatory affairs specialist combines knowledge of scientific, regulatory and business issues to ensure that products are developed, manufactured and distributed to meet regulatory requirements with a focus on the united states and canada. Document your dedication to regulatory affairs for pharmaceuticals, medical devices, and clinical research by earning a professional certification from biopharma institute. Coursework covers international conference on harmonization. Enhance your career with our regulatory affairs certification courses that include practical experience. We offer over forty courses across the topical areas of regulatory essentials, medical devices, pharmaceuticals, quality and clinical that can be taken individually. Gain practical skills and certification to boost your career. Embark on an enlightening journey with our “regulatory affairs specialist” training bundle. Click here to request a quote. Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. Certifications and courses in regulatory affairs significantly enhance job prospects by providing specialized knowledge and skills that are highly valued in the pharmaceutical,. Enhance your career with our regulatory affairs certification courses that include practical experience. Our three ms programs are entirely online: Embark on an enlightening journey with our “regulatory affairs specialist” training bundle. Click here to request a quote. Encrypted pdf with validation qr barcode. The certificate aims to provide participants with regulatory affairs experience for. Four (4) free trial courses are available. The ms in regulatory affairs and quality assurance (raqa); Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. The certificate aims to provide participants with regulatory affairs experience for. Encrypted pdf with validation qr barcode. Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. This meticulously curated selection of courses is tailored to empower professionals navigating the. Enhance your career with our regulatory affairs certification courses that include practical experience. Regulatory affairs specialist combines knowledge of scientific, regulatory and business issues to ensure that products. The certificate aims to provide participants with regulatory affairs experience for. Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. The ms in regulatory affairs and quality assurance (raqa); Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. And. Gain practical skills and certification to boost your career. Join neuage institute's quality assurance and regulatory affairs certification program to gain the essential skills and knowledge for a successful career in the pharmaceutical and biopharma industries. Graduate certificate programs in regulatory affairs are an economical means of training for work in pharmaceutical, medical device, and biotechnology regulatory affairs. Certifications and. The certificate aims to provide participants with regulatory affairs experience for. Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. Our three ms programs are entirely online: Enhance your career with our regulatory affairs certification courses that include practical experience. We offer over forty courses across the topical areas of regulatory essentials, medical devices, pharmaceuticals, quality. The certificate aims to provide participants with regulatory affairs experience for. Encrypted pdf with validation qr barcode. The certificate aims to provide participants with regulatory affairs experience for. Coursework covers international conference on harmonization. The ms in regulatory affairs and quality assurance (raqa); Graduate certificate programs in regulatory affairs are an economical means of training for work in pharmaceutical, medical device, and biotechnology regulatory affairs. Our three ms programs are entirely online: Click here to request a quote. The ms in regulatory affairs and quality assurance (raqa); This meticulously curated selection of courses is tailored to empower professionals navigating the. Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. Gain practical skills and certification to boost your career. Coursework covers international conference on harmonization. This meticulously curated selection of courses is tailored to empower professionals navigating the. Embark on an enlightening journey with. The ms in regulatory affairs and quality assurance (raqa); The certificate aims to provide participants with regulatory affairs experience for. Enhance your career with our regulatory affairs certification courses that include practical experience. Certifications and courses in regulatory affairs significantly enhance job prospects by providing specialized knowledge and skills that are highly valued in the pharmaceutical,. Four (4) free trial. And global clinical and pharmacovigilance. Regulatory affairs specialist combines knowledge of scientific, regulatory and business issues to ensure that products are developed, manufactured and distributed to meet regulatory requirements with a focus on the united states and canada. Certifications and courses in regulatory affairs significantly enhance job prospects by providing specialized knowledge and skills that are highly valued in the. Four (4) free trial courses are available. And global clinical and pharmacovigilance. Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. We offer over forty courses across the topical areas. The ms in regulatory affairs and quality assurance (raqa); This meticulously curated selection of courses is tailored to empower professionals navigating the. And global clinical and pharmacovigilance. The certificate aims to provide participants with regulatory affairs experience for. Encrypted pdf with validation qr barcode. Regulatory affairs specialist combines knowledge of scientific, regulatory and business issues to ensure that products are developed, manufactured and distributed to meet regulatory requirements with a focus on the united states and canada. Join neuage institute's quality assurance and regulatory affairs certification program to gain the essential skills and knowledge for a successful career in the pharmaceutical and biopharma industries. The certificate aims to provide participants with regulatory affairs experience for. Prepare and/or review regulatory submissions to support clinical trial and marketing authorization activities. Meet our renowned instructors in the regulatory writing certificate program to learn more about their experience in the field and the expertise they bring to class. Enhance your career with our regulatory affairs certification courses that include practical experience. Click here to request a quote. Graduate certificate programs in regulatory affairs are an economical means of training for work in pharmaceutical, medical device, and biotechnology regulatory affairs. We offer over forty courses across the topical areas of regulatory essentials, medical devices, pharmaceuticals, quality and clinical that can be taken individually. The us pharmaceutical regulatory affairs program describes the essential requirements for obtaining approval to market a new drug in the usa, the 'nda process'. Document your dedication to regulatory affairs for pharmaceuticals, medical devices, and clinical research by earning a professional certification from biopharma institute.Top Regulatory Affairs Course Drug Regulatory Affairs Certification
Career Counseling Regulatory Affairs
Drug Regulatory Affairs Course [PGDRA] Royed Training
Regulatory Affairs Courses. Regulatory affairs courses are designed
Free Regulatory Affairs Course Entry to Regulatory
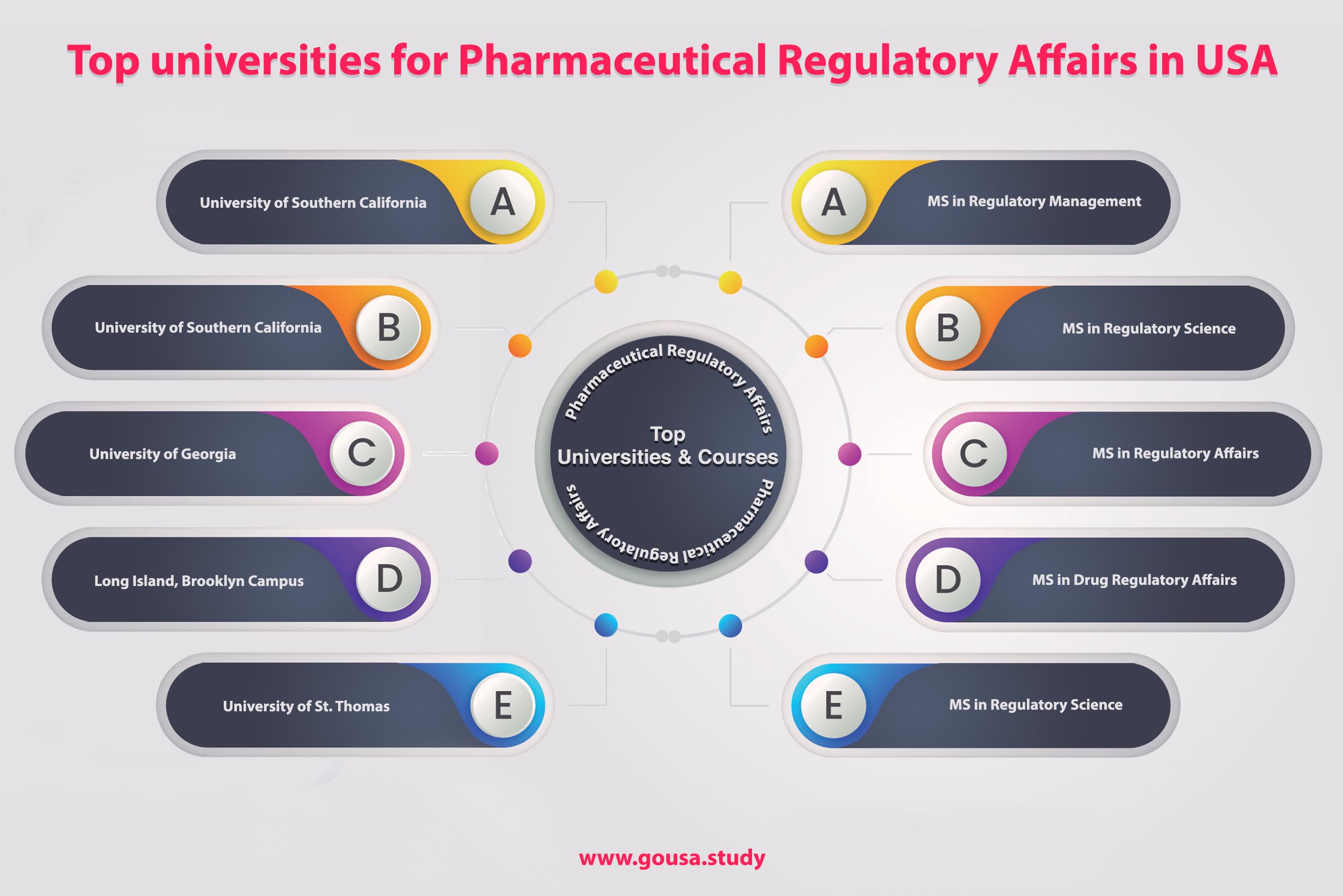
Masters in Regulatory Affairs in USA MS in Pharmaceutical Regulatory
Regulatory Affairs CoursesKickstart Pharmaceutical Career Now!!
Life Science Regulatory Affairs Royed Training
Regulatory Affairs Courses. Regulatory affairs courses are designed
Free Course on Drug Regulatory Affairs with Certificate Free Pharmacy
The Master Of Science In Food Regulatory Affairs Is A Fully Online, Asynchronous Program Tailored For Working Professionals Around The World.
Gain Practical Skills And Certification To Boost Your Career.
Our Three Ms Programs Are Entirely Online:
Embark On An Enlightening Journey With Our “Regulatory Affairs Specialist” Training Bundle.
Related Post:


![Drug Regulatory Affairs Course [PGDRA] Royed Training](https://royed.in/wp-content/uploads/2018/04/drug-regulatory-affairs-course-pgdra.png)